

Catalytic Polymer Re/Upcycling
Plastics are ubiquitous materials produced at a large scale. Plastics are mostly single-use with low recycling rate and degradability, posing an inefficient linear petroleum-to-waste carbon flow, and severe environmental threats. We study the catalysis of converting plastics back into the monomers to achieve a circular economy. We have two projects under this umbrella: 1) tailoring the metal-acid bifunctionality and site proximity of transition-metal-carbide surfaces for efficient impurity-resistant hydrocracking; 2) leveraging precise polymer synthesis, scattering, and spectroscopy methods to decipher the polymer-catalyst interface.
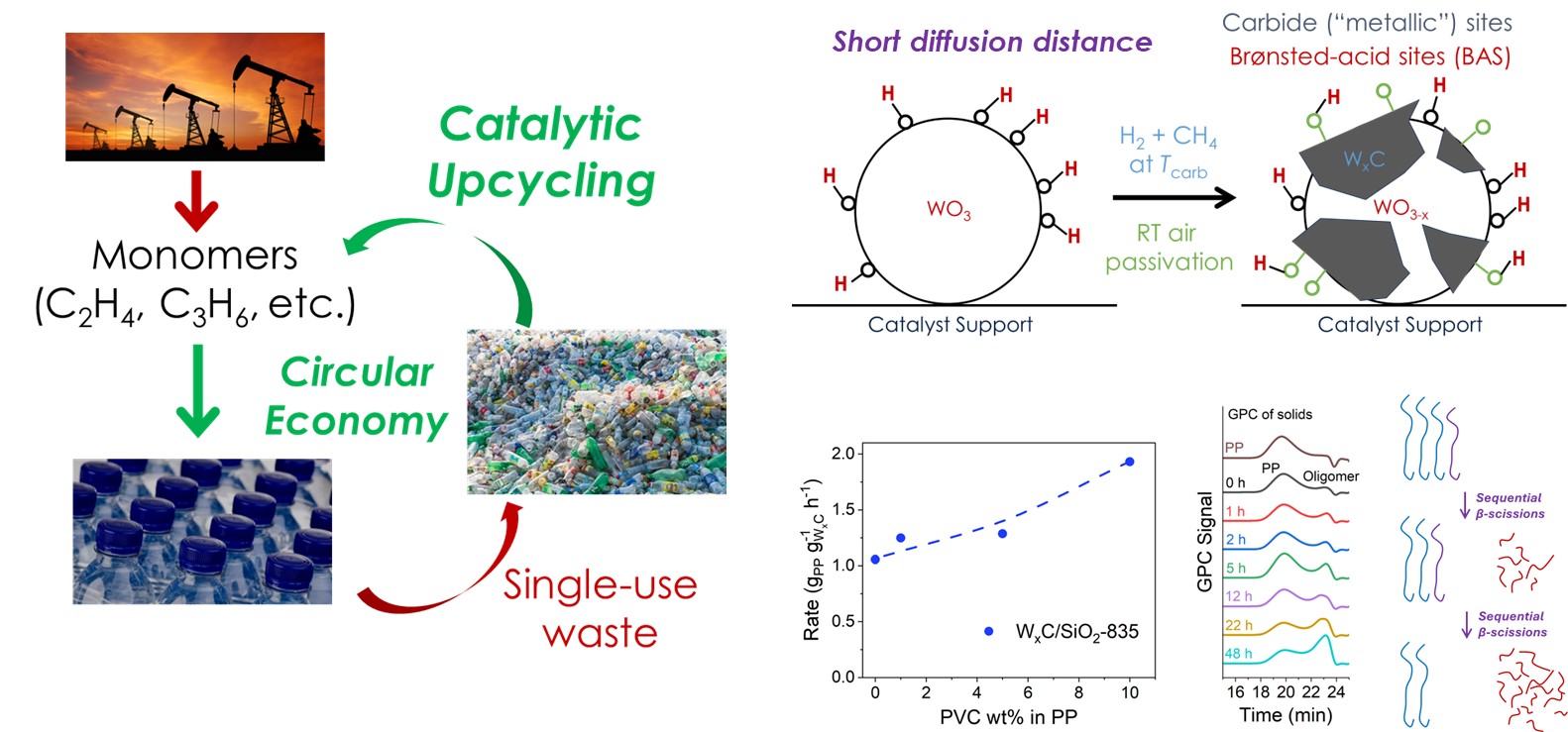
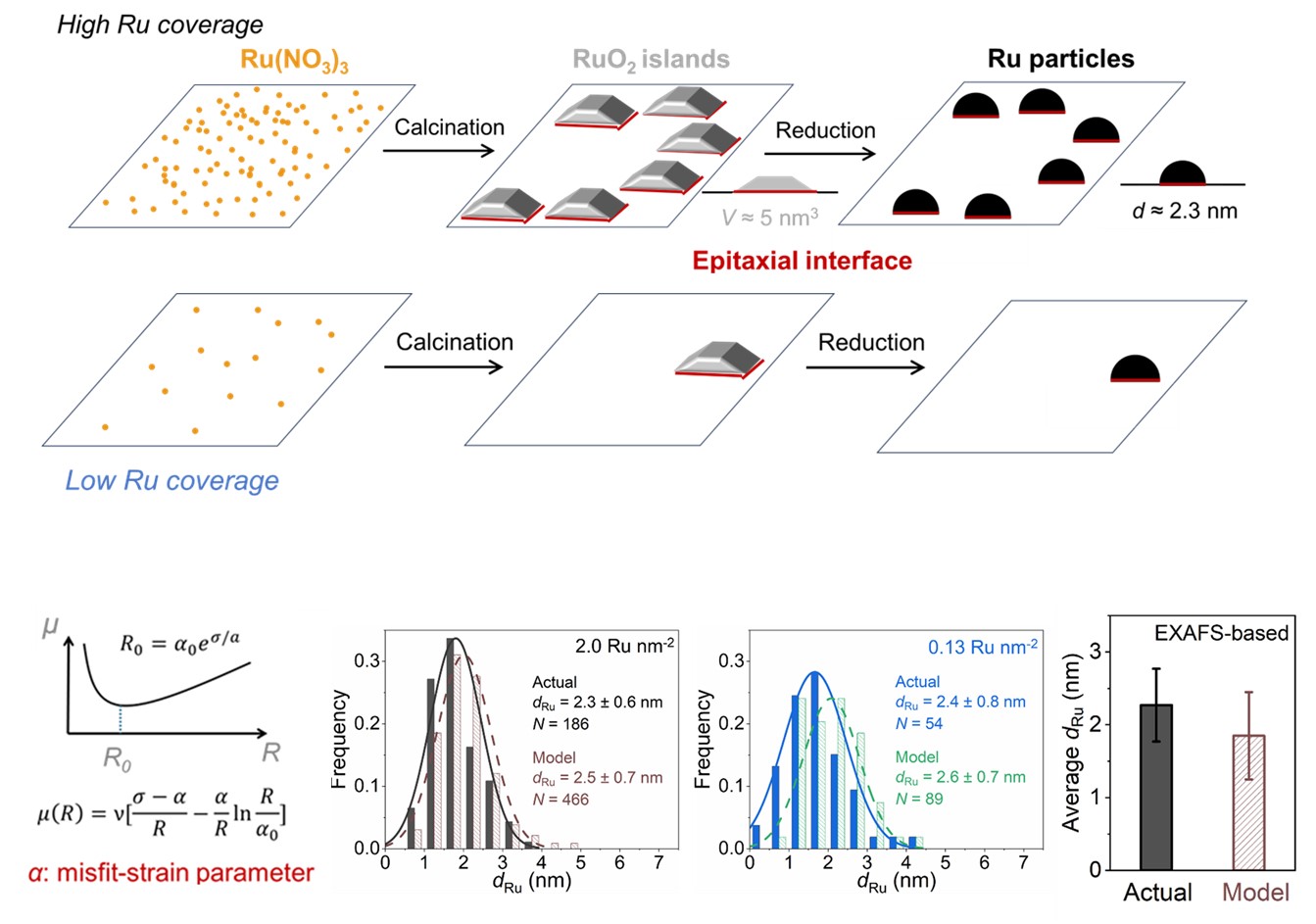
Rigorous Structure-Function Relationship
The performance of heterogeneous catalysts is determined by the size, shape, and density of metal nanoparticles on them. These structural parameters often change simultaneously during synthesis, and thus their catalytic effects (“structure-function relationship”) are difficult to rigorously reveal. We develop an epitaxial-growth-based framework that tunes each structural parameter independently. The platform is deployed to study NH3 decomposition, a reaction for H2-release as clean fuels, from the liquid carbon-free carrier (NH3) that can be safely and easily transported. Structural modelling with comprehensive in-situ characterization is coupled with detailed kinetic analysis to reveal structure-function relationship, building the foundation for future rational catalyst design.
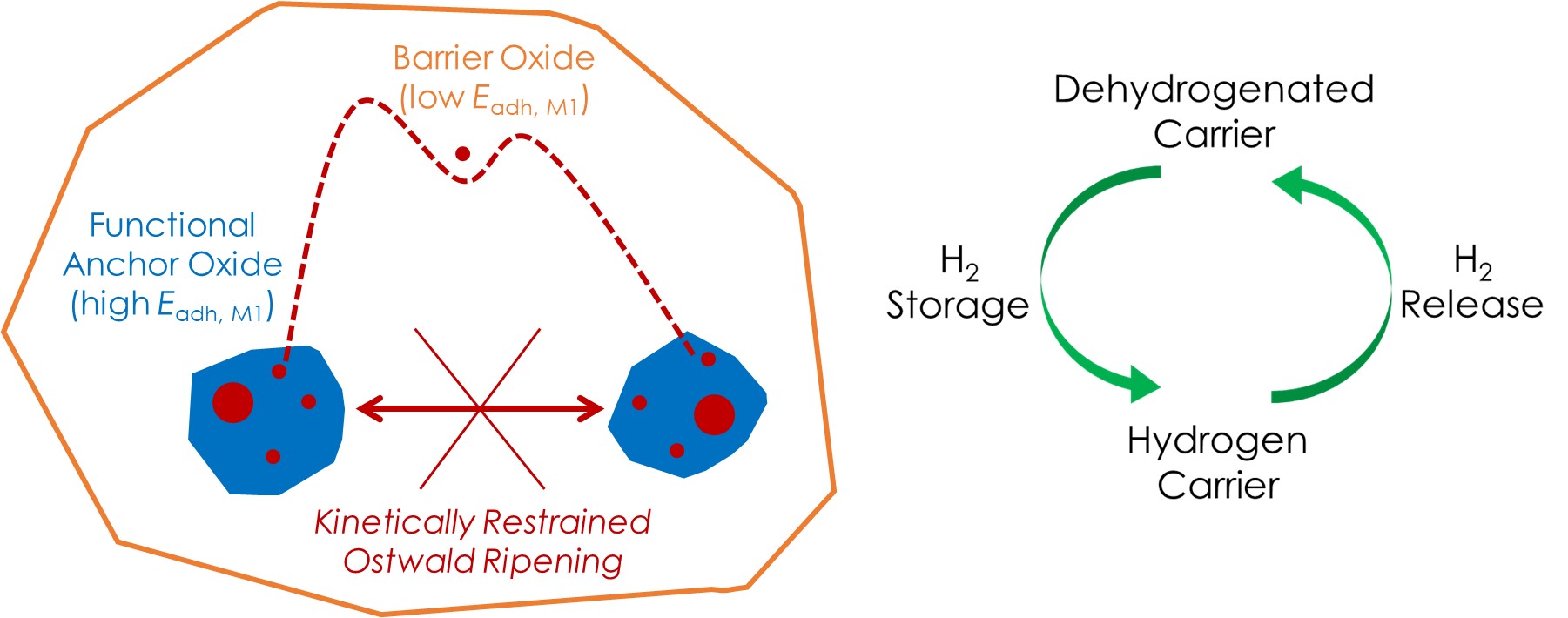
Kinetically Restrained Ostwald Ripening
Low-nuclearity metal clusters (Mn) are desired in catalysis due to the unique electronic properties and structural adaptability. However, the high chemical potential of atoms in Mn leads to strong sintering tendency. We use kinetically restrained Ostwald Ripening to stabilize metastable Mn during high-temperature catalysis. The discrepancy between the adhesion energy of M1 on the anchoring and barrier oxides create interfacial kinetic barriers, restraining Ostwald Ripening to within the anchors. The nuclearity of Mn and surface property of the anchor provide levers for catalytic functionality. We use state-of-the-art characterization techniques to study these systems in-situ, and apply them in sustainability-driven catalysis.
Renewable Carbon-Neutral Chemical
Currently, many chemicals heavily rely on petroleum as the resource. We develop new chemistries and catalysts that convert bio-resources (e.g., lignin) and CO2 into platform molecules at the center of current chemical manufacturing routes (e.g., styrene and ethanol). This will enhance the sustainability and reduce the carbon footprints of the chemicals. We aim at establishing new conversion routes, designing novel catalysts to improve performance, and analyzing footprints of the new processes.
