As a group of chemist-engineer hybrid, our research aims to bridge practical heterogeneous catalysis and fundamental surface chemistry, by studying the three pillars of catalysis science: catalytic materials, spectroscopic characterization, and reaction kinetics. Research activities in the group fall under three inter-connected general themes. Most projects are interdisciplinary and collaborative, involving multiple themes to varying degrees.
Understanding fundamental principes governing heterogeneous catalysis (MAKING SENSE of practical systems)
Heterogeneous catalysis occurs on surfaces with complex structures and harsh reaction conditions. This brings additional challenges to the fundamental understanding of active sites and reaction mechanisms, compared to homogeneous catalysis. We use a wide array of modern in-situ spectroscopy techniques to probe the atomic-level structure of working active sites, and use such knowledge to explain the mechanism indicated by detailed kinetic analysis. We act as “detectives”, to solve puzzles, sometimes unexpected, behind the behaviors of molecules and surfaces during catalysis. While doing so, we often borrow knowledge of model catalysts under clean conditions acquired from surface science and/or theory studies, which are more precise but less relevant to applications.
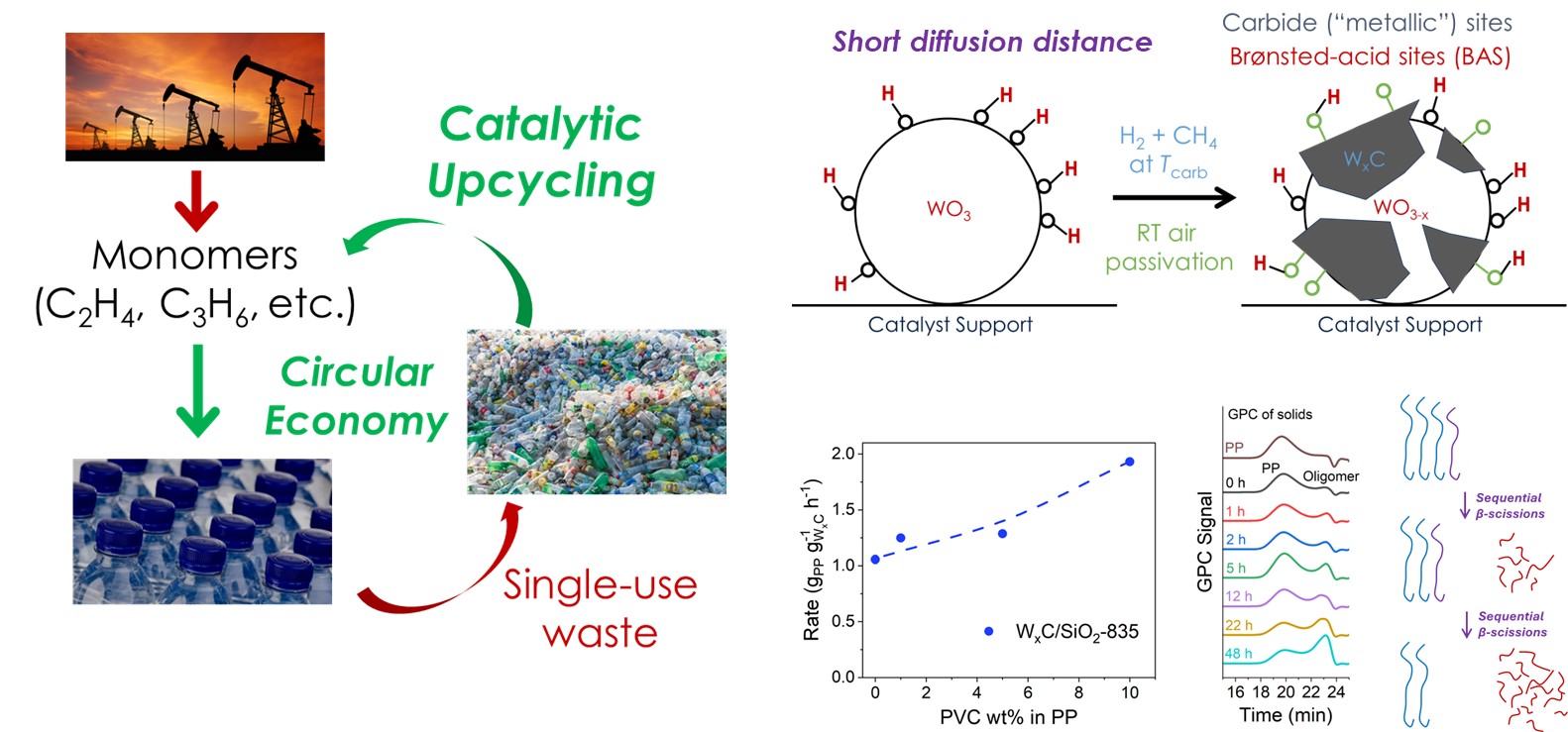
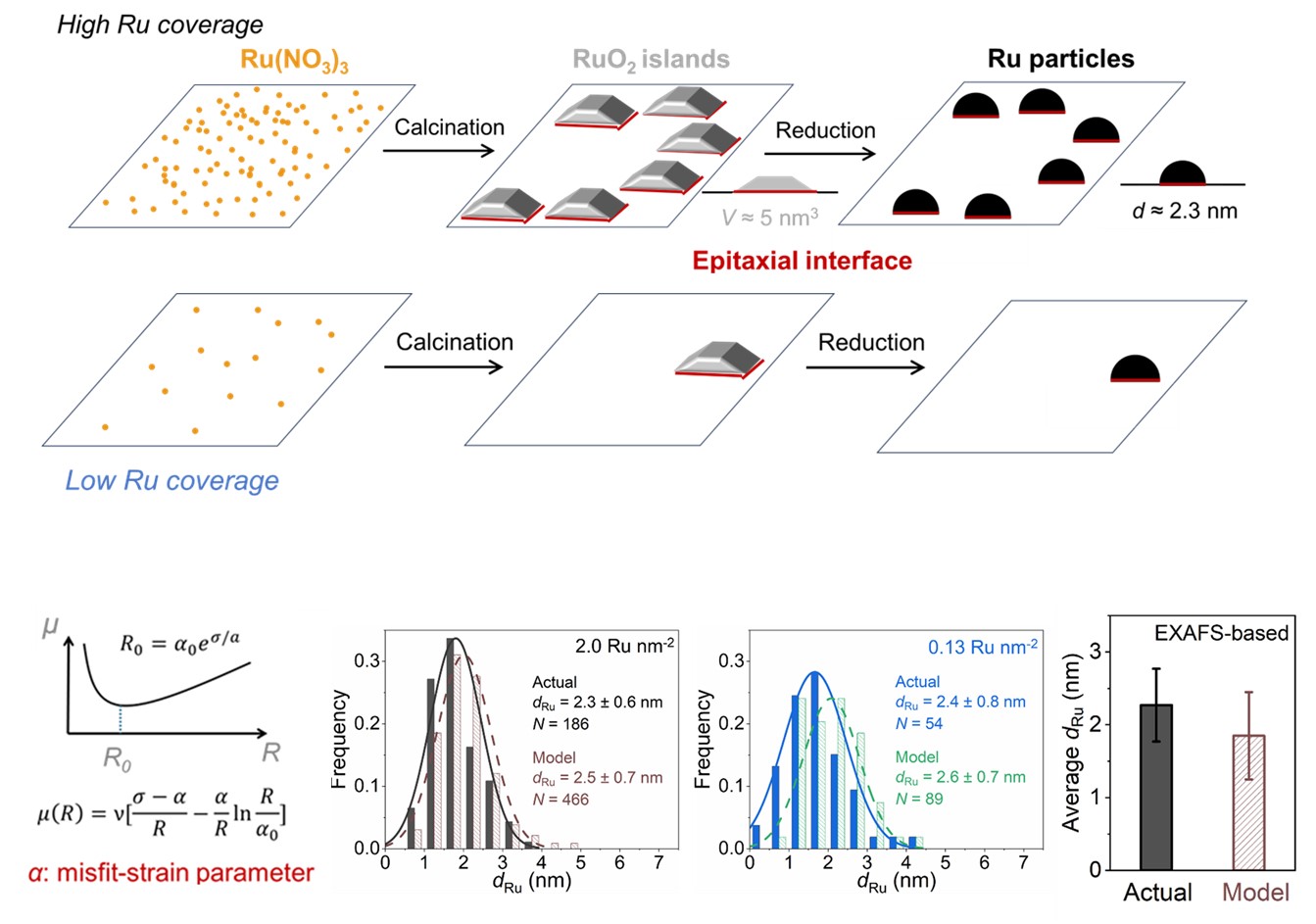
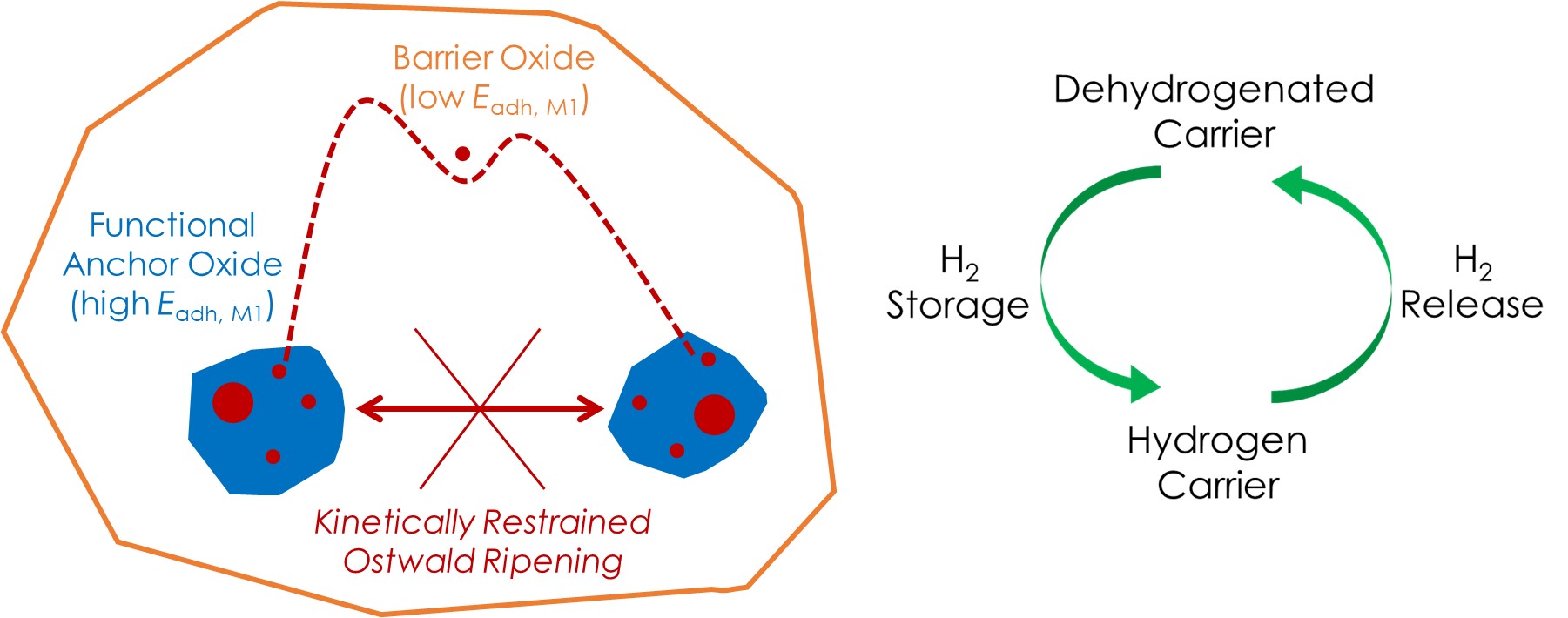
Mechanism-informed catalyst design for sustainability (MAKING USE of fundamental knowledge)
The revolution of modern chemical industry is moving towards sustainable solutions involving renewable feedstocks, lower energy/carbon footprint, and less severe environmental impact. Enabling new chemical routes and/or improving existing ones often require developing new catalysts. Rather than a “pasta-on-the-wall” or “trial-and-error” approach, we emphasize rationally designing catalyst structures, based on how reaction occurs on it, learned by us or others. We sometimes think out of the box by seeking inspirations from surface science, organometallic chemistry, and other scientific fields adjacent (or not) to heterogeneous catalysis. We are interested in broad reaction spaces related to sustainability, such as polymer recycling, hydrogen storage/release, biomass conversion, and carbon circularity.
Method development for catalyst synthesis and characterization
To understand the active-site structure and make new ones in a targeted manner, a versatile toolbox for atomic-level characterization and precise synthesis is needed by us catalysis chemists. Therefore, we constantly look for new ways, often in collaboration with physicists, physical chemists, and materials scientists, to deploy new spectroscopic/synthetic methods to understand/make heterogeneous catalysts. With our broad ties to the national laboratories, this line of work often involves unique “fancy toys” there, such as synchrotron and neutron facilities. We also emphasize “in-situ” methods to acquire structures under catalytically relevant conditions. These efforts are sometimes “organic” (we need a new method to solve a problem), and sometimes intentional (we learn about a capability that we want to explore).